The court today denied two en banc petitions:
Biogen International GmbH v. Banner Life Sciences LLC, 20-1373 (Fed. Cir. 2020)
The law allows for a limited extension of patent term based upon regulatory delays – such as FDA delay in approving a drug for sale. The statute is unfortunately complex and poorly written. 35 U.S.C. 156.
In this case, Biogen received a patent term extension for its Patent US7619001 associated with using the drug Tecfidera (MS treatment). By the time of the litigation, the patent would have expired, but for the term extension.
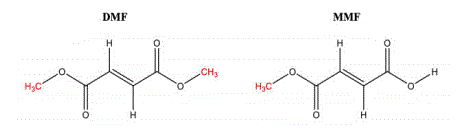
Banner’s proposed version of the drug is a bit different than Biogen’s Tecfidera — although apparently bioequivalent. The court included the following diagram showing the differences with the Biogen’s version is DMF and Banner’s is MMF (both versions were covered by the asserted claim):
In the case, the Federal Circuit held that the term extension did not apply in this situation. The court limited limiting PTE to only situations where the same drug (active ingredient) is being accused of infringement, even if the alternative is covered by the patent claims and includes the same active portion. The court wrote:
Because the scope of a patent term extension under 35 U.S.C. § 156 only includes the active ingredient of an approved product, or an ester or salt of that active ingredient, and the product at issue does not fall within one of those categories, we affirm the judgment of the district court.
In its petition for rehearing, Biogen asked two questions:
- Whether the panel’s decision allows an infringer to avoid an innovator’s patent term extension by using the claimed and bioequivalent active moiety of the compound in the patentee’s approved drug product.
- Whether patent term extension under 35 U.S.C. § 156(b)(2) for method claims is limited to the “approved product” only, where the only limit that Congress imposed on patent term extension for method claims is “any use claimed by the patent and approved for the product,” and Congress further provided that “[a]s used in this subsection, the term ‘product’ includes an approved product” (i.e., is not “limited” to an approved product). 35 U.S.C. § 156(b) (emphases added).
PhRMA also filed an en banc petition in support. The PhRMA brief argues that this decision will allow generic companies skirt the Hatch-Waxman generic-innovator compromise. In particular, generic companies will be able to rely upon innovator data to gain market entry since the active portion of the drug product is the same, while not being subject to the term extension.
The Federal Circuit has denied the en banc petition without further opinion.
[BiogenCAFCDecision] [Discussion by Kevin Noonan]
[Biogen en banc petition] [Biogen PhRMA Amicus]
= = = = =
Dragon Intellectual Property v. DISH Network LLC, 19-1283 (Fed. Cir. 2020).
This is a common setup: Patentee sues for infringement; Defendant collaterally attacks patent via IPR and claims are cancelled; District court then dismisses case as moot – void ab initio. The petition questions whether the defendant can be considered a “prevailing party” for attorney fees in such a situation. “When, if ever, can a litigant be deemed the ‘prevailing party’ in a case terminated as a result of mootness?”
In the case, the district court found no prevailing party in this situation. On appeal, the Federal Circuit rejected that approach – holding that the defendant could be considered a prevailing party because it rebuffed the claims. The Federal Circuit has now denied the en banc petition — meaning that its rule sticks.
"term" - Google News
June 24, 2020 at 05:52PM
https://ift.tt/2Yuj52P
Will this Limit on Patent Term Extension Drive a Rewriting of Hatch-Waxman? - Patently-O
"term" - Google News
https://ift.tt/35lXs52
https://ift.tt/2L1ho5r
Bagikan Berita Ini

















0 Response to "Will this Limit on Patent Term Extension Drive a Rewriting of Hatch-Waxman? - Patently-O"
Post a Comment